Neurons and cancer cells are a dangerous duo
New research finds that neurons migrate from the brain to infiltrate cancer cells, and that targeting this process is a promising new method of attack on cancer.
Oleg Tsupykov on Wikimedia Commons
Scientists have thoroughly studied the connections between the central nervous system and other systems, because our brain controls pretty much everything in our body: our movements, our emotions, and even our gut. Now, researchers are starting to realize the brain might also control something much more sinister - cancer.
Malignant tumors are groups of abnormal cells that can divide quickly, spread, and ultimately develop into cancer. In 2001, cancer biologists discovered the presence of nerve fibers—the projections that come from neurons—in tumors. Although neurons are typically regarded as harmless or perhaps even beneficial, this turned out not to be true in tumors. It appears that tumors can co-opt the signals neurons produce in order to grow, such as growth factors and neurotransmitters—hinting that neurons and cancer cells together might be a dangerous duo.
While the relationship is still being explored, studies across many different types of cancer suggest the presence of neuronal connections in tumors may be detrimental. For example, a high number of nerve fibers are often present in the tumors of patients with prostate cancer. The number of nerve fibers correlates with worse clinical outcomes, suggesting that the more neuronal connections there are in a tumor, the more aggressive the cancer.
Understanding why tumors develop these dangerous neurons in the first place could help identify new forms of treatment. Since the initial observation that cancer severity is associated with nerve fibers, cancer biologists have been searching for the source of tumor-invading fibers and neurons. Finally this spring, a study published in Nature found a very surprising answer.
The study, led by Claire Magnon at the Institute of Cellular and Molecular Radiation Biology in France, examined prostate cancer patients. The researchers discovered that a specific type of neural progenitor cells were present in the patients' prostates. These cells are identified by the genetic marker doublecortin (DCX+). The more DCX+ cells, the quicker the tumor grew and the more widely it spread. Next, Magnon's team looked at various mouse models of prostate cancer. Again, in the prostates of diseased mice they found an accumulation of DCX+ neural progenitor cells that were absent in healthy mice. Clearly, neuronal cells were infiltrating or being produced in the tumors.
This turned out to hold true for other types of cancer, too. By studying a mouse model of breast cancer, Magnon's team again found that neural progenitor cells accumulated in tumors. The researchers began to suspect that recruitment of neurons may be a general process for cancer development. But where were the cells coming from?
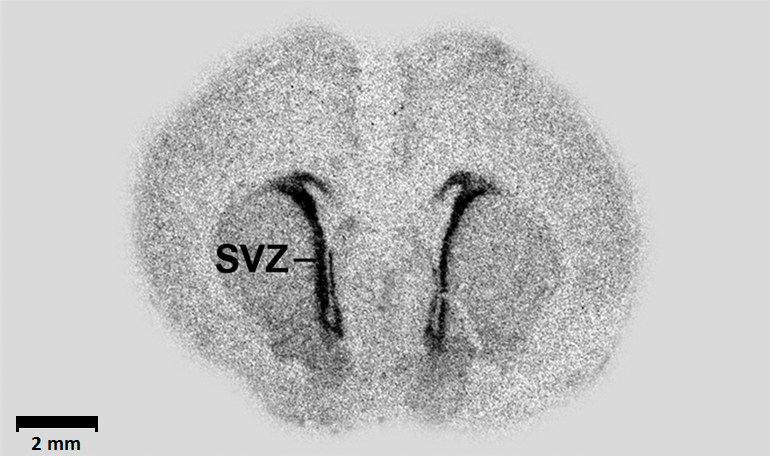
Autoradiograph of the SVZ from a rat brain
Neural progenitor cells are normally only present in very specific areas of the brain, including the subventricular zone (SVZ). Looking at these areas in the prostate cancer mouse model, the authors saw that these cells in the SVZ—but not anywhere else in the brain—decreased throughout cancer progression. When the scientists started tracking the SVZ during cancer development in mice, they realized that the neural progenitor cells actually left the brain, migrated through the blood, and infiltrated the tumor!
As a neuroscientist who has learned that neurons are very fragile and need a lot of nurturing, this was surprising to me. How do neural progenitors get out of the brain, travel such a huge distance, and survive inside a tumor? Magnon's co-author Philippe Mauffrey was able to start answering the first of these questions. Magnon's team observed that in the SVZ, where neural progenitor cells decreased during cancer, there seemed to be a breach in the barrier that separates the brain from the rest of the circulatory system. Nowhere else in the brain did they detect a similar disruption. This breach likely allowed the cells to escape out of the brain and into the body at large.
It's truly surprising that after getting out of the brain, neurons would be able to survive and travel all the way to a tumor. We also do not know what would trigger them to leave the SVZ in the first place. The next piece of the puzzle is figuring out what pushes the neural progenitor cells to venture into the unknown. The most popular hypothesis at the moment is that the tumor itself may be producing signals that attract the neural progenitor cells.
But it is now clear that once the neural progenitor cells get to the tumor, they elicit neoneurogenesis, or the birth of new neurons within the tumor. This in turn creates a microenvironment where cancer cells can thrive. Logically, Magnon's team decided to get rid of the environment that would help cancer cells grow by simply deleting the neural progenitor cells. This significantly inhibited tumor development in the prostate cancer mouse model.
Other studies have taken different approaches with similar outcomes. For example, a study published in Science found that removing the connections by neurons in mouse models of gastric cancer reduced tumor incidence and progression and increased survival rates. Another study from Johns Hopkins University found that blocking a specific neurotransmitter, a chemical that neurons use to communicate with each other, similarly halted tumor growth. These results suggest that intervening in the crosstalk between the central nervous system and tumors may be a new therapeutic target.
Studying how neurons infiltrate tumors, like this bundle of colon cancer cells, may be a new avenue into treating cancer
NCI Center for Cancer Research on Flickr
Unfortunately, messing around with the brain and the nervous system can be a little tricky. In humans, controlling cancer progression will not be as easy as deleting neural progenitor cells from the brain, because this could have other, less desirable impacts. Although Magnon's study did not examine the effects the deletion of neural progenitor cells had on mouse behavior, I suspect it could have detrimental effects on cognition. For example, in research by David Greenberg's lab from the Buck Institute for Research on Aging, deletion of these same DCX+ neural progenitor cells worsened stroke outcome in mice. Therefore, possible interventions will have to be careful to avoid affecting brain function negatively.
It remains to be seen how relevant these findings are to humans, but they do point the field of cancer biology in a new direction: Go to the brain to find the source of neuronal cells that fuel cancer growth. Hopefully this is only the beginning—studying these neurons and how they infiltrate tumors may provide keys for how to better monitor, predict, and potentially treat cancer.
Peer Commentary
Feedback and follow-up from other members of our community
Sruthi Sanjeev Balakrishnan
Cell Biology
National Centre for Biological Sciences
This is a beautifully written article, Claudia! The finding that neurons and cancer cells have an unfortunate tie-up is something that has generated quite a buzz in recent times. I am particularly intrigued by the fact that there is a migration of progenitor cells from the SVZ. Therapeutic potential aside, what does this mean for an untreated animal?
Does the reduced progenitor number in the SVZ impair regular neuronal functions in animals suffering from prostate cancer? According to the authors, the SVZ progenitors later become interneurons in the olfactory bulb. Would this mean that, as the cancer progresses, olfactory capabilities of the mice reduce?
The study cites a review that summarises cognitive defects seen in cancer survivors (both CNS and non-CNS type cancers). However, this review only looks at the effect of chemo/radiotherapy on cognition, most likely because it was published before the link between tumors and neurons came into light. I think it might be worth looking into the cognitive effects of these neuron-tumor links first in untreated patients. This might help in designing therapeutics that can circumvent any possible neuronal side-effects.
Thanks Sruthi! I had only thought of it in terms of a therapy, but I agree-there must be consequences due to the decrease of neural progenitors in the brains of untreated animals with cancer that should be explored, especially in the context of the developing brain. I think olfactory capabilities could be affected, but more importantly there would also probably be decreased plasticity and decreased neural repair in response to injury. As for humans, they really have no way to confirm whether or not this decrease is also occurring in their brains (although maybe they could look at the blood, since they are suggesting the cells travel through the circulation?) But I would say the next step would be to study the effects on neuronal function and cognition in the cancer mouse models and then go into untreated cancer patients, as you say.
I think another controversy comes into play which is, is there even significant neurogenesis in the adult brain? A recent study showed there is, but results have been mixed. However, if significant neurogenesis does not occur in the adult brain, then maybe this decrease in neural progenitor cells wouldn’t really have an effect on neuronal function or cognition. A lot to be explored here!
Alyssa Shepard
Cancer Biology
The Scripps Research Institute
I think this is a nice article on an interesting and complex topic. It’s really not too surprising to see research supporting a neural network in tumors, seeing as more and more research has come about showing the importance of the tumor microenvironment and how tumors might be more of an organism than a random clump of cells. I’ve been reading a lot lately about the link of evolution and cancer - there’s evidence that cancer cells act similarly to single-celled organisms, so the hijacking of neuron progenitors might contribute to the idea that tumors are almost separate from our normal tissues, almost using us as a host (a terrifying and disgusting thought, in my mind).
Anyway, I’ll be interested to see where the research leads on this. Specifically, the migratory mechanisms of the progenitors to the tumors. That’s what I might be most doubtful on at this stage, even though they have shown preliminary evidence of tracking the specific cells through the blood-brain barrier. In my mind, the tumors could be hijacking more localized neurons (perhaps peripheral sensory neurons) and altering them to a more progenitor-like state. But then again, I’m not a neuroscientist, so I could be completely off base! Overall, good job explaining a complex article!
Alice Theibault
Environmental Science, Biotechnology
Rochester Institute of Technology
This article is fascinating! It seems like over time there’s been newer, less-conventional targets for reducing tumor growth. I’d previously known that researchers were looking into how bacteria interact with tumor cells, but it’s interesting to see a role for neurons as well.
Jacqueline Mattos
Plant Ecology, Ecology & Evolutionary Biology
Federal University of São Carlos
Impressive article! It was really surprising for me that the neural progenitor cells really migrate from the brain to the tumor, enabling its growth. I was intrigued by the fact that, for treatment, you would have to delete the cells from the human brain, which then you said could lead to other unwanted impacts. I believe that this is a nice path to keep following in cancer research, but sometimes I am concerned with those kind of possible outcomes from digging too much in other “unaffected” areas of the body. What are your thoughts on that? Thank you for the well written article and please keep us informed on the outcomes of this field!
Bhavya Singh
Microbiology
McMaster University
This poses a very interesting question. Instead of being viewed as our own cells going haywire, could cancers instead be almost like parasitic organisms? I’m a microbiologist, so I’m not too familiar with the cancer research field, but this is just absolutely fascinating. I wonder that when neurogenesis is elicited, what kind of neural cells are being formed around the tumor, and if we see the same kinds of neural cells growing alongside different kinds of tumors? Not going to lie - this is a bit terrifying!
Kevin Pels
Chemical Biology
Dana-Farber Cancer Institute
It’s worth mentioning that this phenomenon may be consistent with only a subset of cancers, as the type of cells they worked with (PC-3) are similar to a histology of prostate cancer called neuroendocrine prostate cancer (NEPC), unlike other commonly used prostate cancer cell lines LnCAP or DU145. Compared with other/earlier stages of prostate cancer, NEPC has a much worse prognosis because it is a more aggressive tumor that is not dependent on androgen signaling (anti-androgens are the first lines of therapy in prostate cancer). So perhaps beyond NEPC, the subset of tumors recruiting neuronal involvement or hijack nearby nerves to support oncogenesis is indicative of aggressiveness, but also provides a therapeutic opportunity. I found a recent review (http://cancerdiscovery.aacrjournals.org/content/9/6/702) that indicates a potential role for cardiovascular drugs called beta blockers that block adrenergic signaling used by SNS neurons, which are now in multiple clinical trials for a few different cancers.
Vanessa Vieites
Psychology
Florida International University
I really appreciated this thorough and accessible article written by Claudia. I like how it includes a helpful video on the neuron for readers who aren’t familiar with neuroscience, mentions other studies outside the original one, links to such studies to back up claims with empirical evidence, and is careful to mention the studies were done on rodents and that it isn’t clear yet how the findings mentioned in the article are relevant to humans (very important when discussing animal work). As someone who values nuance (and, of course, accuracy) in science writing, thank you, Claudia, for the responsible reporting.