This 3D-printed ovary could help the women left out of modern fertility technology
We're a long way from a human version, but the initial results are promising
One morning in lab, a small group of us gathered to discuss the use of a new technique in our field for growing tissues on a dish, but we ended up contemplating a newly published paper describing the creation of a bioprosthetic ovary.
“It looks like a raindrop cake” said Stephen, our rotation student. We quickly googled it; raindrop cakes are a thing.
“It’s like a jello salad with pomegranate seeds,” observed Holly, a postdoc in the lab.
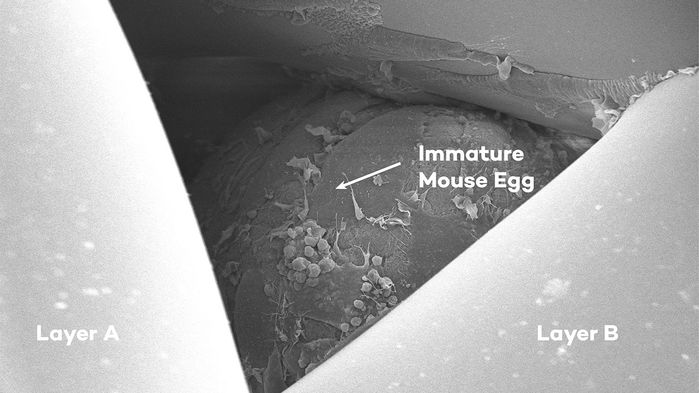
In immature mouse egg sits in a scaffold
Northwestern University
We may have differed on the point of comparison, but we all agreed: the technology we were discussing was delicious in its innovative scientific properties: a group of scientists created an ovary made of a porous scaffold seeded with ovarian follicles – small pockets made of cells where oocytes, or egg cells, reside. When they implanted these bioprosthetic ovaries in sterile mice, some of these mice gave birth to pups.
That is to say, the artificial ovary worked. This is potentially game-changing news for an overlooked demographic in the rise in fertility technology: women with damaged ovaries.
In the last few years, big tech companies have started covering the (high) costs of egg freezing for their female employees. While the current procedures (oocyte and embryo cryopreservation) are suitable for healthy women, people whose ovaries were hurt by previous illnesses, or the treatments they underwent to cure them, are out of luck.
Ovarian transposition and tissue cryopreservation are viable alternatives for some women, but they have their limitations: the first one is mostly used before radiation therapy, surgically moving the ovaries outside the radiation field to avoid their exposure; the second one is considered experimental and consists of freezing the whole ovary, or a part of it, to preserve it until implanting it back it in the patient.
Neither of these are a safe option for organs damaged by certain diseases such as leukemia. If the disease is characterized by the presence of cancer cells in the blood, for example, freezing the whole tissue carries the risk of freezing and then implanting back in the cancer cells as well.
Because of the flaws of these procedures, scientists started investigating alternatives to solve or bypass different issues. When the development of the bioprosthetic ovary started, there were already published studies on the growth of ovarian follicles supplemented with vascular and growth factors and embedded in a special matrix to mimic the ovarian matrix. Some of these studies even led to birth from the cultured follicles.
But the bioprosthetic ovary presents an 'upgrade' of these milestone techniques. The main idea is a whole functioning organ that would allow women in the future to have multiple pregnancies without having to go through the process currently required for IVF or implantation. Moreover, this idea aims to be a long-term solution and has more value than we may think: the ovary is an endocrine organ that secretes steroid hormones, estrogens, and progesterone, with important systemic functions.
So it’s not all about fertility here: hormones are required for the woman’s overall health. Several studies have linked the loss of hormone production to conditions such as osteoporosis, cardiovascular, and psychological diseases. These conditions are also more frequent in post-menopausal women. A brand new ovary could re-establish the hormone balance lost in women who underwent early menopause.
The authors got to work, deciding to take advantage of 3D printing, which allows for control of key parameters, such as pore design for diffusion. Unfortunately, the authors didn’t find many published studies describing the ideal geometry and configuration for their needs. So they experimented.
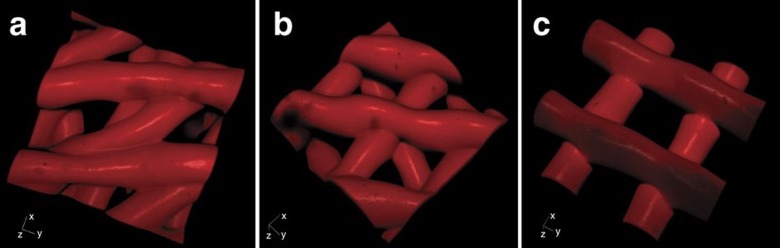
Scaffolding at 30º (a), 60º (b) and 90º (c)
The first quandary was choosing the right material to use as ink for 3D printing. They decided on a gelatin-like material similar to collagen, a component present in the matrix of both human and mouse ovaries. The next step was how to prepare the scaffold for the follicle. They tested three designs characterized by distinct advancing angles with each layer (30°, 60°, and 90° – the optimal angle was 60°), that provided different physical support to cells: more contacts with the struts of the scaffold kept follicles in their spherical shape (fundamental for follicle survival), and the cells were capable of proliferating, and adhering to the matrix in the same fashion they do in vivo.
At this point, everything seemed to properly work in vitro – in a dish. The consequent step was moving in vivo, implanting the bioprosthetic ovaries in mice whose ovaries were surgically removed.
The implanted follicles expressed a green fluorescent marker (green fluorescence protein, GFP), so they were easily traceable and, if a pup were born from one of the bioprosthetic ovaries, he’d be green when exposed to a special light. Amazingly, of the seven mice that received the implants, three were able to give birth to one or two (green) pups after mating, and were able to feed them through natural lactation, demonstrating that the ovaries were secreting the appropriate hormonal levels. But it doesn’t end here: once the pups reached adulthood, they gave birth or sired their own litters.
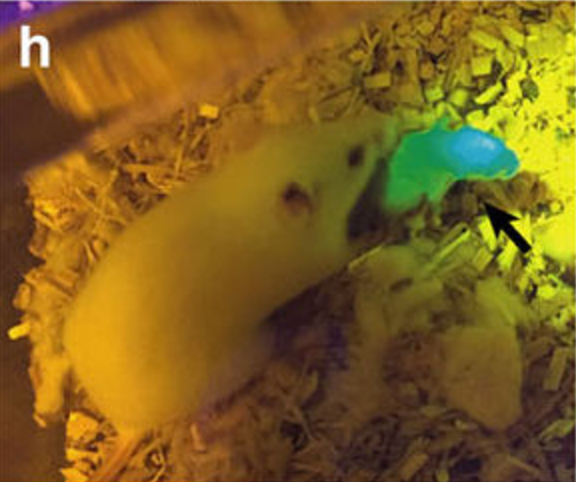
A mice pup from one of the prosthetic ovaries glowing green
The big question is whether this is scalable to humans. We still don't know how long the implant is going to last in the body of a mouse, much less in the human body. Moreover, in cases of oncofertility, there is still the need to obtain follicles to seed in the bioprosthetic ovary that don't contain cancer cells.
The bioprosthetic ovary won't be an option available for people anytime soon. But when it is, many women and girls that will be facing or already had illnesses that compromised their ovaries will have an opportunity to carry out a life that for most of us is blessedly normal. Some diseases leave patients with the feeling of having lost control of their bodies, and what this paper describes is another step forward in the field of regenerative medicine to give back patients that lost sense of ownership back.